Glue, the magical sticky stuff that holds our world together, has always left us scratching our heads. Is it a liquid? A solid? Or maybe even a gel? This mind-boggling question has sparked endless debates throughout history. But fear not. In this blog post, we’re diving deep into the captivating world of glue to unravel its enigmatic nature and discover if it truly deserves the liquid label.
When we think of liquids, we imagine them flowing freely, taking on any shape their container allows. Solids, on the other hand, hold their ground with unwavering rigidity. So where exactly does glue fit into this puzzle? In its liquid form, glue behaves like your typical liquid. It pours smoothly, adapting to every nook and cranny it encounters. But here’s where things get interesting – over time, it undergoes a remarkable transformation. Gradually solidifying into a formidable bond that refuses to let go.
Sure, glue shares some similarities with everyday liquids like water or oil. But it possesses an undeniable uniqueness all its own. Unlike water that slips away and evaporates without a trace, glue sticks around for the long haul. It clings tenaciously to surfaces and hardens upon exposure to air, forging unbreakable connections. This chameleon-like quality allows glue to bridge gaps, fill in cracks, and ensure lasting unions.
The versatility of glue is a testament to its liquid-like qualities. From fixing shattered ceramics and sealing wooden masterpieces to assembling intricate models and rescuing household items from certain doom – there’s nothing this adhesive wonder can’t do. Its ability to bind different materials together showcases its true nature as a liquid adhesive with special powers that set it apart from ordinary liquids.
Venturing further into the realm of adhesives reveals an entire spectrum of glue forms – pastes, gels, and even aerosols. In these variations lies the true essence of glue, blurring the lines between solids, liquids, and gels. But fear not, dear reader. Despite this confusion, we can still classify glue as primarily a liquid due to its remarkable flowability at room temperature and its gradual solidification over time.
In this captivating blog series, we’ll embark on an adventure through the molecular composition of glue, its manufacturing secrets, and its practical applications. We’ll uncover the science behind its liquid-like properties and compare it to other adhesives in the
What is Glue?
Contents
- 1 What is Glue?
- 2 The Components of Glue
- 3 Is Glue a Liquid?
- 4 Glue’s Properties as a Liquid
- 5 How Glue Transforms into a Solid State
- 6 Different Types of Drying Mechanisms for Glue
- 7 Is Glue Considered a Non-Newtonian Fluid?
- 8 Conclusion
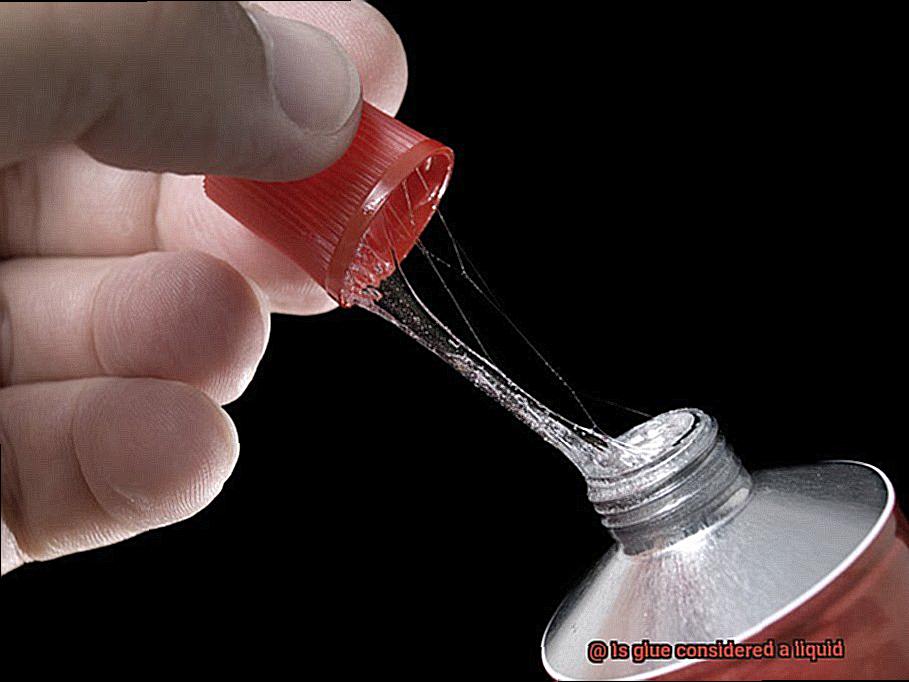
When it comes to sticking things together, glue is the unsung hero. This remarkable substance, available in liquid, gel, or solid form, has the power to create unbreakable bonds. But what exactly is glue, and how does it work its magic?
At its core, glue is a carefully crafted combination of polymers, solvents, and additives. Polymers, those mighty molecules made up of repeating subunits called monomers, are the backbone of glue. They provide the adhesive properties that allow glue to create strong bonds between surfaces. Popular polymers used in glue include polyvinyl acetate (PVA), epoxy, and cyanoacrylate.
The solvent in glue serves a crucial purpose by making it easy to apply. Solvents are the magical liquids that dissolve the polymer and give glue its spreadable consistency. Once applied, the solvent evaporates, leaving behind a solid adhesive bond. The choice of solvent depends on the type of glue and its intended use.
But there’s more. Additives are like secret ingredients that enhance the performance and properties of glue. These substances can boost adhesion strength, flexibility, drying time, or resistance to moisture and heat. Fillers, plasticizers, thickeners, and preservatives are just a few of the common additives found in glue.
Glue can be classified into different types based on its composition and properties. Water-based glues use water as their solvent and are known for being non-toxic and easy to clean up. Solvent-based glues rely on organic solvents like acetone or toluene and offer stronger bonding properties. Then there are hot melt glues: solid at room temperature, but they melt when heated and form a bond upon cooling.
When using glue, it’s vital to understand its unique properties. In liquid form, glue flows freely to fill gaps and adhere to different materials. But unlike ordinary liquids, glue undergoes a transformative process when it dries. The solvent evaporates, leaving behind solidifying polymers that create a sturdy and long-lasting bond.
The Components of Glue
Glue, that unsung hero of sticking things together, is a remarkable substance with a carefully crafted combination of polymers, solvents, and additives. Polymers, the backbone of glue, provide strength and elasticity. Animal-based glues use collagen from bones and hides, while plant-based glues can be derived from starch or cellulose.
Solvents or water make glue spreadable. Solvents dissolve the polymers and other ingredients in glue, making it easier to apply. Solvent-based glues use organic compounds as solvents, while water-based glues obviously use water. Water-based glues are often safer and more environmentally friendly.
Additives enhance glue’s performance. Plasticizers increase flexibility and reduce brittleness. Fillers improve consistency and strength. Preservatives prevent microbial growth, and stabilizers keep everything in check.

Different types of glues may have slightly different compositions based on their intended applications. Wood glue contains resins or waxes for better adhesion to wooden surfaces, while fabric glue incorporates substances that resist washing or dry cleaning.
Is Glue a Liquid?
Glue, the wondrous substance that binds our world together. But is it truly a liquid? Let’s dive into the sticky truth and uncover the secrets of this remarkable adhesive.
At first glance, glue appears to be a liquid. It flows effortlessly from its container, spreading like a molten river across surfaces. Its viscosity allows it to penetrate even the tiniest cracks and crevices, ensuring a strong and lasting bond. But here’s the twist – glue is not your ordinary run-of-the-mill liquid.
When freshly applied, glue indeed takes on a liquid form. It’s like a superhero in disguise, ready to save the day. In this state, the liquid glue can be easily shaped and molded to fit our needs. It’s the opportune moment to piece together that jigsaw puzzle or mend that broken chair leg.
But wait. Just like Cinderella’s carriage turning into a pumpkin at midnight, glue undergoes a fascinating chemical reaction called curing or drying. Brace yourselves, for this is where the real magic happens. As glue dries, it undergoes a transformation from liquid to solid, leaving us awe-struck witnesses to this enchanting process.
So why does this happen? The answer lies in the composition of glue itself. Glue is made up of polymers, solvents or water, and additives. Polymers provide strength and flexibility, while solvents or water give glue its liquid characteristics. And those secret weapon additives? They enhance performance and durability.
As the glue is applied and exposed to air, the solvents or water begin to evaporate. This evaporation process causes the polymers to draw closer together, intertwining like an intricately woven tapestry. And just like that, the once flowing liquid transforms into a solid material – a testament to its powerful bonding capabilities.
But hold on. The time it takes for glue to fully cure can vary depending on the type of glue used and environmental conditions. So, exercise patience, my friends. Allow the glue to work its magic and metamorphose from a fluid to a solid state.
In conclusion, while glue starts off as a liquid, it ultimately undergoes a remarkable transformation into a solid material once it has fully dried. So, the next time you grab that trusty bottle of glue, remember the sticky truth – it’s a liquid superhero waiting to transform into a solid savior.
Glue’s Properties as a Liquid
Today, we embark on a deep dive into the remarkable properties of glue in its liquid state. Get ready to be amazed as we explore how this liquid superhero wields its adhesive powers.
Penetrating Power:
Glue’s liquid state grants it a superpower – the ability to penetrate even the tiniest gaps and irregular surfaces. Like a secret agent infiltrating enemy lines, glue seeps into every nook and cranny, ensuring a bond that can withstand the test of time.
Conforming to Shapes:
Imagine a shape-shifting superhero who can mold themselves to fit any situation. Well, glue does just that. Its liquid nature allows it to conform perfectly to the shape of the objects being bonded. Whether it’s jewelry or a broken vase, glue bends and molds itself to ensure a secure and snug fit.
Easy Application:
With its low viscosity, glue is incredibly easy to apply and work with. Its liquid state allows for precise application and control, so you can target specific areas without any wastage. It’s no wonder that crafters, artists, and DIY enthusiasts all rely on this versatile tool.
Viscosity Variations:
Not all glues are created equal. Each type of glue possesses its own unique viscosity, catering to specific applications and materials. From runny superglue to thick wood glue, there’s a glue for every job, ensuring optimal bonding results.
Sticky Magic:
Ever wondered why glue is so sticky? It’s all thanks to its liquid state. In its liquid form, glue is naturally tacky, allowing it to adhere and hold surfaces together. Think of it as your trusty sidekick that keeps everything firmly in place.
How Glue Transforms into a Solid State
Prepare to be captivated as we delve into the scientific marvel behind this extraordinary metamorphosis. Step into this adhesive journey with us, and witness the magic of transformation unfold before your eyes.
The Dance of Evaporation and Bonding:
The Liquid State:
In its liquid form, glue is a symphony of polymers dissolved in a solvent. This concoction possesses low viscosity and natural tackiness, allowing it to effortlessly spread and cling to surfaces.
The Evaporation Enigma:
As the solvent evaporates, the glue embarks on its wondrous journey towards solidity. This evaporation exposes the polymers to the air, triggering a dazzling display of chemical reactions and physical bonding.
Polymer Power:
During this awe-inspiring transformation, the polymers unite and interweave, creating an unyielding adhesive bond. The specific combination of polymers employed determines the unique characteristics of different glues, such as flexibility or strength.
Factors Influencing the Speed of Transformation:
Climate Control:
The elements of humidity and temperature wield their influence in determining the pace of glue’s metamorphosis. Higher humidity slows down evaporation, while warmer temperatures accelerate the process, expediting the bond formation.
The Waiting Game:
The time required for glue to solidify varies from one type to another. Some glues boast rapid drying times, achieving their full strength within minutes, while others demand patience, requiring hours or even days to reach their pinnacle of solidification.
The Diverse Mechanisms:
Wood Glue’s Secret:
Wood glue possesses a clever trick up its sleeve. It absorbs moisture from the air to cure and solidify. This ingenious mechanism ensures a tight grip on wood surfaces, forging bonds that can withstand the test of time.
Superglue’s Rapid Transformation:
Superglue, the hero of swift fixes, undergoes a rapid polymerization process. Upon contact with moisture, it instantly solidifies, forming an unbreakable bond in the blink of an eye. Its lightning-fast action has made it a staple in our toolkits.
The transformation of glue from a liquid to a solid state is nothing short of extraordinary. Witnessing its adaptability and its ability to forge unyielding connections between materials fills us with awe. So, the next time you reach for that bottle of glue, marvel at the magical journey it undertakes to become your steadfast ally in all your crafting and DIY endeavors.
Different Types of Drying Mechanisms for Glue
Glue is a remarkable substance that possesses the ability to bind objects together. However, the process by which glue dries and solidifies is often overlooked. In this article, we will explore the different drying mechanisms for glue and their effects on the strength and durability of the bond formed. Let’s delve deeper into the fascinating world of glue drying.
Solvent-Based Glue:
Solvent-based glues, also known as solvent adhesives, rely on the evaporation of a solvent to dry and form a bond. These glues contain solvents such as acetone or toluene, which dissolve the adhesive and allow it to spread easily. As the solvent evaporates, the glue undergoes a chemical reaction called polymerization. During polymerization, the adhesive molecules bond together, transforming the glue from a liquid into a solid bond. The rapid evaporation of solvents in solvent-based glues results in quick drying times.

Water-Based Glue:
Water-based glues, or emulsion adhesives, use water as their primary solvent instead of harsh chemicals. These glues are considered more environmentally friendly and safer to use. The drying mechanism for water-based glues involves the evaporation of water and the coalescence of polymer particles. As the water evaporates, the polymer particles come closer together and form a solid bond. Due to the slower evaporation rate of water, water-based glues generally have longer drying times compared to solvent-based glues.

Heat Curing:
Some glues require heat to initiate their drying process. Heat-curable adhesives are commonly used in industrial applications where high strength and durability are imperative. When heat is applied to these adhesives, a chemical reaction is triggered within the adhesive, causing it to harden and form a strong bond. Heat curing can be achieved through various methods, such as hot air, infrared radiation, or direct contact with a heat source. The application of heat allows for faster drying times and ensures a reliable bond.
UV Curing:
UV-curable adhesives contain special photoinitiators that react when exposed to ultraviolet light. When the adhesive is exposed to UV light, the photoinitiators initiate a chemical reaction that causes the adhesive to harden and form a bond. UV curing is widely used in industries such as electronics, optics, and automotive, where quick drying and high bond strength are crucial. The advantage of UV curing is its ability to provide instant drying, allowing for increased productivity and efficiency.
Is Glue Considered a Non-Newtonian Fluid?
Glue, the adhesive that binds our world together, has long fascinated us with its sticky magic. But does it fall into the category of non-Newtonian fluids? Prepare to delve into the scientific realm of glue and uncover the truth.
To understand this query, let’s first grasp the essence of non-Newtonian fluids. These substances defy Newton’s law of viscosity, meaning their viscosity can change under different conditions. However, glue does not exhibit such behavior.
Non-Newtonian fluids come in various types, like shear-thinning and shear-thickening fluids. Shear-thinning fluids, such as ketchup and certain paints, become less viscous as the applied force increases. Conversely, shear-thickening fluids, like a mixture of cornstarch and water, become more viscous when force is applied. Yet, glue does not fit into either of these categories.
There is also a class of non-Newtonian fluids called viscoelastic fluids that possess characteristics of both solids and liquids. Silly putty and oobleck (a cornstarch-water mixture) are examples of viscoelastic fluids. But alas, glue does not join their ranks.
Glue maintains its relatively thick and consistent nature regardless of the force exerted upon it. It exhibits no significant changes in viscosity when subjected to varying shear rates or stresses. Thus, based on its behavior and properties, glue cannot be considered a non-Newtonian fluid.
Yet fear not. Though glue may not fall under the non-Newtonian umbrella, it possesses its own enchanting qualities that make it an indispensable tool in our daily lives. When it dries, it solidifies and forges strong bonds between materials. Different types of glue employ various mechanisms to achieve this transformation.
Solvent-based glues rely on evaporation to dry—when the solvent evaporates, it leaves behind the adhesive material that creates the bond. Water-based glues, on the other hand, coalesce polymer particles as the water evaporates, forming a solid bond.
There are even heat and UV curing methods wherein glue is exposed to heat or ultraviolet light, initiating a chemical reaction that results in a hardened bond.
_w94grcw808″ >
Conclusion
In conclusion, it is clear that glue is indeed considered a liquid.
Its ability to flow and take the shape of its container makes it meet the definition of a liquid. Whether it’s in the form of a bottle or a tube, glue has the characteristic properties of a liquid.
It is viscous, sticky, and can be poured or spread easily.
You may also like:








